
While sulfur is mostly present in the form of H2S, other sulfur species can be found in significant concentrations in distinct regions, such as:
These compounds significantly vary in concentrations worldwide.
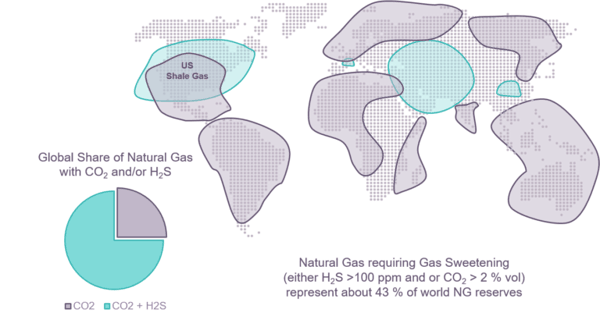
Figure 1 - Localization of sour natural gases containing H2S as sulfur contaminant
When present, the concentration of sulfur compounds, especially H2S, varies from a few ppm up to several vol % or even for the most sour gas to several tens of vol %. In order to avoid corrosion and toxicity issues and to meet the transport and end users specifications of natural gases the sulfur species are extracted, usually with solvent process, and recovered as an acid gas stream rich in H2S. According to the composition and flowrate of the raw natural gas feed, this acid can represent hundreds, sometimes, thousands, tons per day of sulfur that has to be recovered.
The H2S present in those acid gas streams can be valorized by oxidation to sulfur through Sulfur Recovery Unit by being transformed into elemental sulfur (Sx). In turn, elemental Sulfur is used in the fertilizer and rubber industries, and for the extraction of some metals (copper, nickel, uranium), mainly via its intermediate conversion into Sulfuric Acid (H2SO4).
According to CRU group (Sulfur Market Outlook, March 2019, CRU Group), annual world sulfur demand would represent about 65 million tons in 2019. This demand is deemed to increase by 12% by 2023. As of today, natural gas is a steady source of sulfur and provides for half of its demand. Achieving high recovery rates in those Sulfur Recovery Units, is essential to meet today’s environmental standards, be it in refineries or in natural gas plants.
After the Gas Sweetening process, the extracted H2S rich gas is referred as ‘Acid Gas’, since H2S is an acidic component. It is sent to the Sulfur Recovery Unit (SRU). In this Unit, sulfur is generated in gaseous phase by oxidation with air, as per Figure 2. It is recovered as a liquid after condensation, and can be either shipped as is, or solidified into bright yellow particles to ease its transportation.Recovered Sulfur is then feeding the global sulfur market.
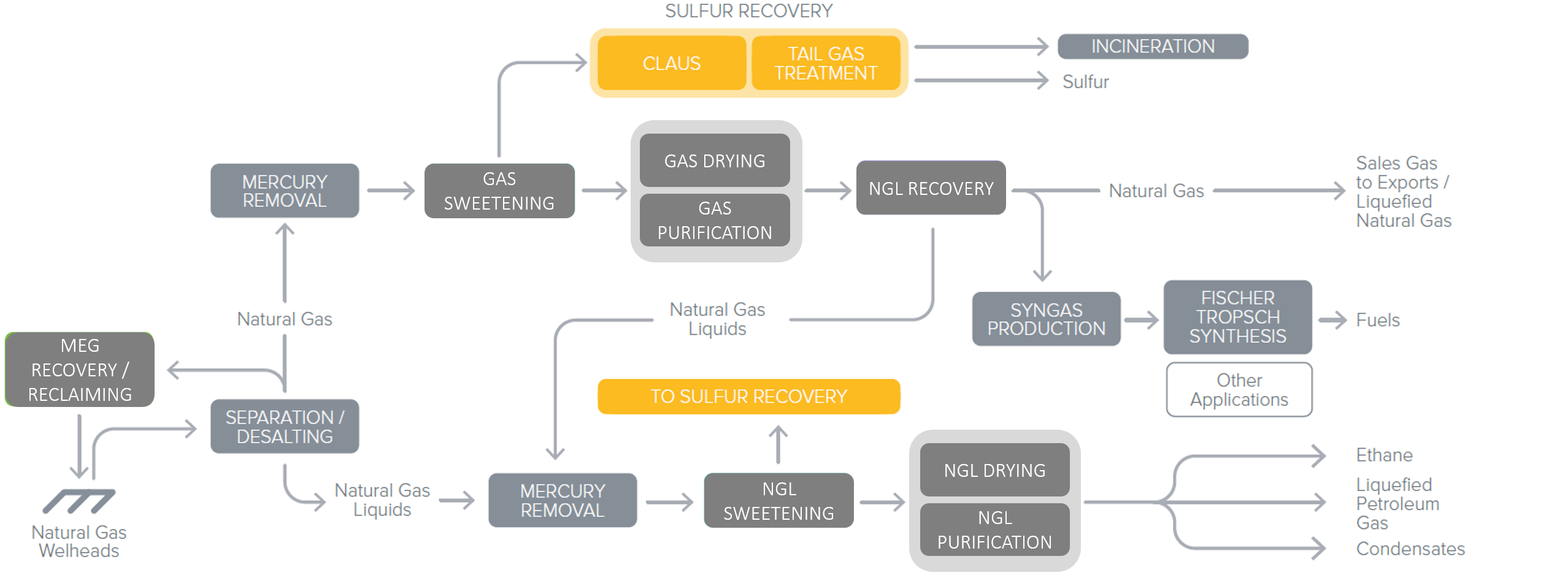
Figure 2
Axens offers a comprehensive portfolio of advanced process solutions and products for Sulfur Recovery with suitable design for gas plants, refineries, upgraders and biogas. Axens technologies and products are well suited to efficiently convert H2S into elemental sulfur (Claus or SmartSulf™ processes) and to meet the most stringent environmental standard (with Tail Gas Treatment process Sultimate™).
The Claus and other advanced process solutions for sulfur recovery involve using thermal and catalytic reactions. Sulfur recovery can range from 95% up to 99.5+% (with SmartSulf™) depending on the process used. Sulfur recovery yields can be further boosted beyond 99.9% by the addition of Tail Gas Treating Units like Sultimate™.
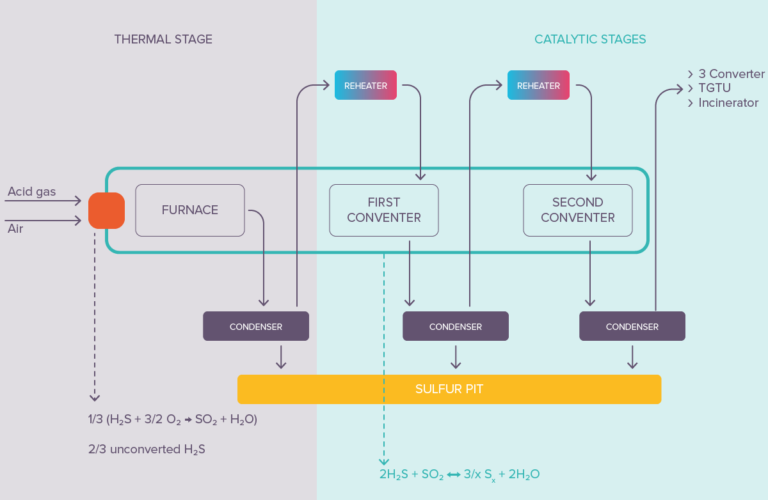
Figure 3
In the catalytic stages (after the initial thermal stage) of the sulfur recovery unit, the formation of sulfur occurs at the surface of heterogeneous (solid) catalysts, mainly Claus alumina. The first activated alumina ever introduced into a sulfur recovery unit in 1957 was produced in our Salindres plant.
The key to sulfur recovery maximization is then to avoid pore blockage in the catalyst. Large pore openings are required to facilitate the diffusion of gases in direction of the active surface sites, but more importantly to allow the evacuation of the produced large S8 molecules out of the catalyst. High macroporosity and ultra-macroporosity are key performance indicators.
When dealing with lean acid gases (containing high concentrations of CO2) some carbonyl sulfide (COS) and carbon disulfide (CS2) are generated in the reaction furnace. Loading titanium-based catalysts in the first catalytic reactor, alone or in combination with alumina, is the solution of choice (invented by Axens in 1982) to allow the recovery of the sulfur element entrapped in these species and reach outstanding performances.
For extra lean acid gases, Axens has developed a unique promoted alumina that enables dealing with the adverse effect of aromatics on the catalyst cycle duration.
For the Tail Gas Treating Units, Axens developed a generation of hydrogenation catalysts able to be operated at low inlet temperature (down to 200°C at reactor inlet), so that customers can save energy and operating costs. For new units, these catalysts allow installing steam reheaters instead of the traditionally used direct-fired burners, preventing sooting events from occurring. These catalysts have been widely accepted by the market.