Aug 18 2020
9 min read

Gamma-alumina’s defect structure, surface acidity and high surface area make it extremely useful as an adsorbent, catalyst and catalyst support. The critical stage in its manufacture as a catalyst support for naphtha reforming catalysts lies in the control of its specific surface area and the concomitant distribution and activity of its inherent acid sites. A combination of calcination conditions and subsequent treatments sets these dependent variables during alumina manufacture.
Nanometer-sized gamma-alumina crystallites are the foundation of all modern naphtha reforming catalysts, comprising more than 95 wt% of their composition.
FIG. 1 contains a depiction of a perfectly formed gamma-alumina crystallite, the smallest grain that contains gamma-alumina’s structure, and a TEM image of actual gamma-alumina crystallites used to produce naphtha reforming catalyst. The 3-digit Miller indices, given in parentheses, represent the orientation of the crystallite’s crystallographic planes. Abutting crystallites bind together through Al-O-Al linkages to form larger porous aggregates with diameters ranging between 30 nm and 500 nm. These aggregates pack together to form even larger agglomerates with diameters ranging between 5 μm and 10 μm.
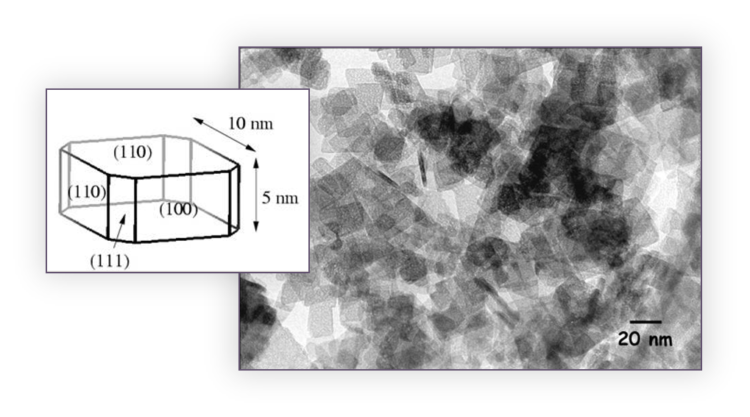
FIG. 1 – Depiction of a perfectly formed gamma-alumina crystallite and TEM image of gamma-alumina crystallites used to produce naphtha reforming catalyst
The crystallites, aggregates and agglomerates together determine the pore size, pore size distribution and surface area of the material. Pores result from the voids between the nanometer-sized crystallites and between the larger aggregates and agglomerates. Macropores and mesopores serve as inter-aggregate highways and inter-crystallite streets, respectively. This bimodal pore system provides efficient reactant ingress and product egress, and impacts catalyst activity and deactivation rate.
Gamma-alumina’s surface provides three primary contributions to naphtha reforming catalyst:
As depicted in FIG. 2, hydroxyls on the catalyst surface serve as weak Brönsted acid sites with acid strength inversely proportional to the O-H bond strength. Chlorine addition pulls electron density away from the O-H bond, making the H sufficiently acidic to catalyze the desired naphtha reforming molecular rearrangement reactions: chlorine promotes the acidity. On average, each nm2 of surface area provides up to ten potential Al-OH or Al-Cl sites. A fresh catalyst with 180-m2/g specific surface area and 1 wt% Cl content contains about one Al-Cl site and 1–4 Al-OH sites per nm2 of available surface area, depending on the operating conditions.
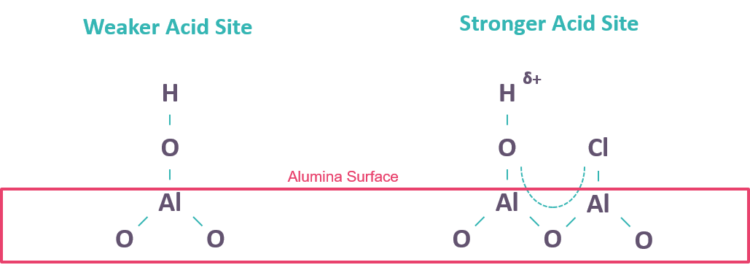
FIG. 2 – Depiction of a weaker Al-OH Brönsted acid site and a stronger Cl-promoted Al-OH acid site
As shown in FIG. 3, a crystallite’s surface can contain imperfections. These imperfections increase the material’s specific surface area, but can be temporary. In the case of metastable gamma-alumina, diffusion of its surface aluminum atoms will remove these imperfections to minimize surface energy and lower surface area.
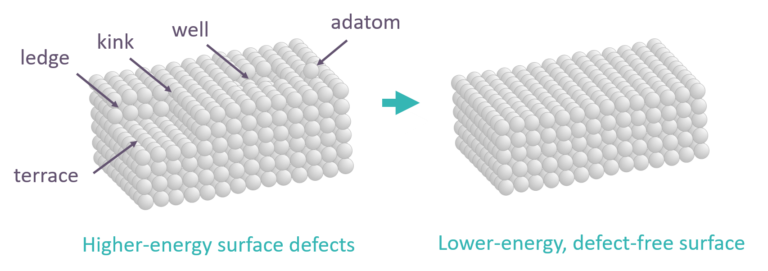
FIG. 3 – Depiction of crystallite imperfections and outcome of their removal
FIG. 4 provides a simple analogy to help understand the relative magnitude that defect removal has on a crystallite’s specific surface area and size. Assume that a golf ball is a crystallite and its dimples are defects. The specific surface area and radius of the golf ball can be calculated from the provided information. Recalculation of the specific surface area and radius for a defect-free sphere containing the same quantity of material shows that the specific surface area has dropped by approximately 30% while the radius has dropped by a little more than 2%. For reforming catalyst, this result implies that specific surface area loss from defect removal has little impact on gamma-alumina crystallite size, and the associated pores should become slightly wider.
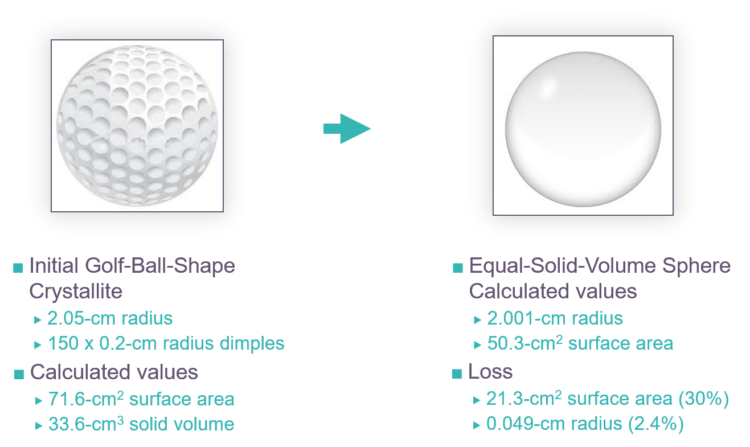
FIG. 4 – Depiction of crystallite imperfections and outcome of their removal
As shown in FIG. 5, increased moisture and temperature lower gamma-alumina’s specific surface area stability. Moisture reacts with the gamma-alumina surface to reversibly create neighboring surface hydroxyls (Al-O-Al + H2O ↔ 2Al-OH). Neighboring surface hydroxyls can also condense to dehydroxylate the surface. Diffusion of surface Al atoms results from the breakage and reestablishment of surface Al-O-Al bonds, since the two Al-OHs can recombine with other available neighboring Al-OHs. As a result of this mechanism, the rate of specific surface area loss is proportional to (PH2O)1/2: a fourfold increase in water partial pressure will double the rate of specific surface area loss. Increased temperature accelerates this surface diffusion. In reforming, catalyst specific surface area loss occurs primarily during catalyst regeneration when temperature and water partial pressure are both elevated.
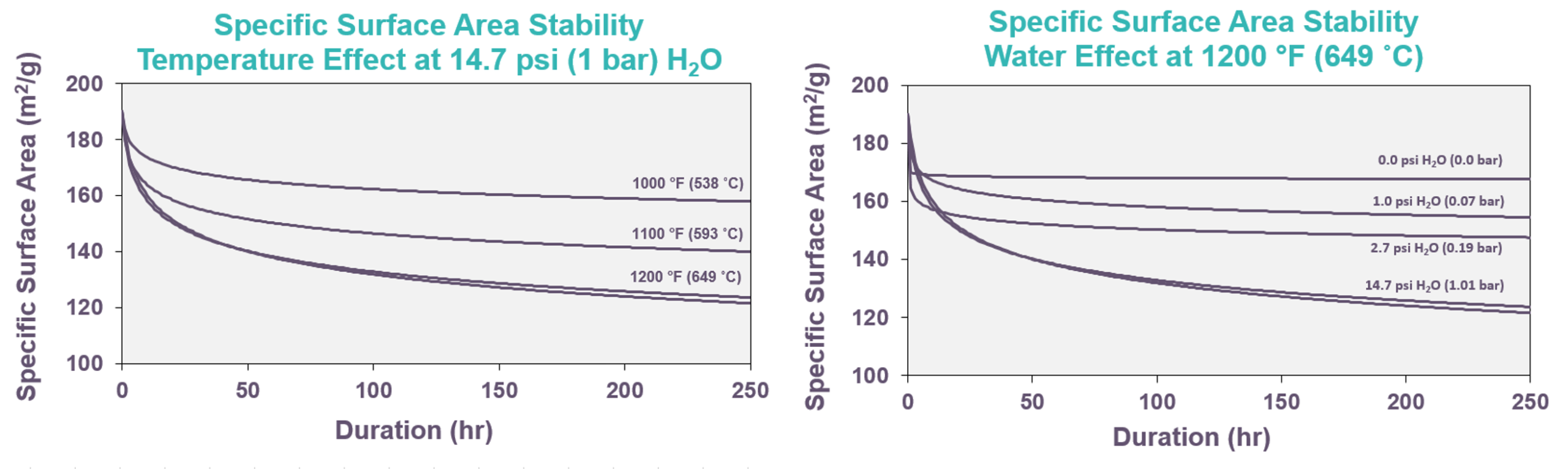
FIG. 5 – Temperature and water partial pressure impacts on gamma-alumina’s specific surface area stability
Fractional specific surface area loss due to surface atom diffusion at constant conditions is represented by the following equation:
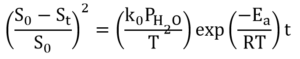
Where:
t: Exposure time
S0: Specific surface area of fresh catalyst
St: Specific surface area of catalyst after t exposure time
k0: Rate constant for specific surface area loss
PH2O: Water partial pressure
T: Temperature
Ea: Activation energy for specific surface area loss
R: Gas constant
For a given reformer with relatively constant regeneration severity and duration, fractional surface area loss can be expressed by the following simplified equation.
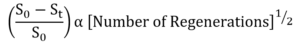
FIG. 6 illustrates shows how well this equation represents the fractional surface area loss for a commercial CCR catalyst that has undergone about 150 regenerations. The slope of this equation varies with regeneration severity and duration, causing different units to fall on their own respective lines for the same catalyst, as shown in FIG. 7.
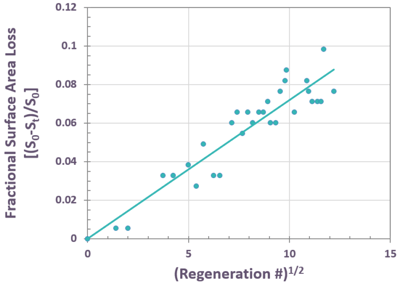
FIG. 6 – Fractional specific surface area loss for a single CCR reformer
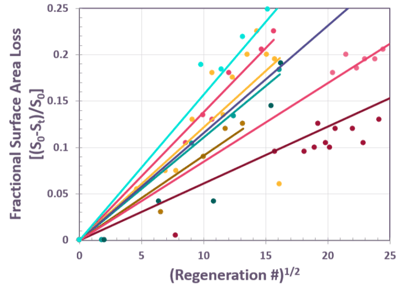
FIG. 7 – Fractional specific surface area loss for multiple CCR reformers with the same catalyst
Loss of specific surface area directly impacts naphtha reforming catalyst performance. The number of potential Al-OH and Al-Cl surface sites drops in proportion to this loss. Fewer potential sites for Al-Cl necessitates a higher density of surface Al-Cl to maintain constant catalyst Cl content, which necessitates injecting more Cl-containing species into the regenerator, feed or both. Higher Cl injection rates directly translate to higher Cl losses in the process and regenerator effluents.
Less specific surface area brings the Pt crystallites into closer proximity, increasing the probability of their agglomeration. Pt clusters containing more than 13 Pt atoms may not afford full Pt availability. Redispersion of agglomerated Pt clusters becomes more challenging as surface area declines, especially if catalyst Cl content is not maintained. Ample Pt availability is essential to initiate and terminate the most desirable reforming reactions.
Besides providing the acid function and a platform for the Pt crystallites, the surface area also serves as a “parking lot” for coke, as well as some permanent alumina poisons. A smaller parking lot lowers the coke threshold for the onset of catalyst performance loss. It also magnifies the impact of poisons (K, Na, Mg, Ca, Si, S, etc.), since these occupy one or more of the alumina surface sites—and may hinder others—further reducing the catalyst’s effective surface area.
The indirect impacts of specific surface area loss are numerous and include:
TABLE 1 lists results from a pilot plant test of a fresh CCR catalyst (~190 m2/g) and two lower specific surface area catalysts (~160 m2/g and <140 m2/g) created by steaming the fresh catalyst for different durations. The two steamed catalysts underwent a pseudo regeneration to redisperse the Pt crystallites and establish the same catalyst chlorine content prior to testing. All three catalysts were tested using the same feed and high-severity operating conditions to achieve accelerated deactivation. The same on-stream water and chlorine addition protocols were practiced for all three catalysts. Reactor temperature was adjusted independently for each catalyst to maintain the same octane severity.
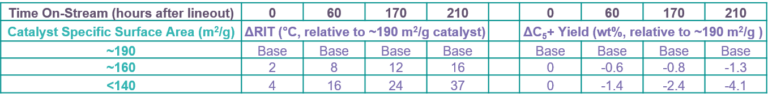
TABLE 1 – Pilot-plant-measured impact of specific surface area loss on CCR reformer catalyst performance
Despite the large difference in specific surface area, the two lower-specific-surface-area catalysts initially exhibited similar performance to their higher-specific-surface-area parent at the start of the test: the same C5+ yield was achieved with only a slight decline in activity for the lower-specific-surface-area catalysts. This performance similarity soon vanished as the test progressed. C5+ yield and activity declined far more rapidly for the two lower-specific-surface-area catalysts. The magnitude of this deterioration increased progressively for each reduction in catalyst specific surface area.
The loss of catalyst performance associated with the loss of its specific surface area directionally results from the following mechanism. For constant water and chlorine make-up, catalysts with lower specific surface area retain less chlorine. The resulting loss in chlorine content for the lower-specific-surface-area catalysts reduces their activity and necessitates operation at higher temperature to maintain target octane. Higher temperature operation accelerates coke formation and concomitant performance loss. The lower-specific-surface-area catalysts have less surface area (a smaller parking lot) to accommodate coke, which reduces the coke-content threshold for performance loss. A lower coke threshold coupled with accelerated coke production amplifies performance loss.
Specific-surface-area stability enhances naphtha reforming catalyst performance, prolongs its useful lifetime and reduces downstream corrosion and fouling.
Axens is one of the world’s largest suppliers of aluminas for catalysts and adsorbents. It marries ultra-high purity raw materials with superior technical expertise developed over the last 150 years to manufacture naphtha reforming catalysts with exceptional specific surface area stability. These catalysts maintain fresh-like catalyst performance well beyond the competition.