Jun 15 2022
17 min read

Summary
What are RON & MON? ➜ C5/C6 Isomerization in a Nutshell ➜HCl Recovery Innovation by Axens ➜ Licensees Testimonials ➜ Takeaways and Video ➜ |
Gasoline specifications trends are governed both by environmental standards and by the targeted energy efficiency of car motors.
Gasoline quality is thus targeting cars low toxic emissions and high anti-knocking property (ignition quality).
The reaction desired in a car combustion chamber is a rapid but gradual ignition, and not an instantaneous one. If the air-gasoline mixture-burns much faster than the crankshaft is rotating, the explosion, also called knock phenomenon, causes a high-pressure rise in a smaller volume (because the piston has not moved far enough), with possible piston damage as a consequence.
Historically, since the 1930s’, knock resistance is measured with Research Octane Number (RON) and Motor Octane Number (MON) (ASTM D-2699 and D-2700, respectively) standard tests. The analyses consist in a comparison between the sample and a blend n-heptane (Octane Number =0) of highly reactive long linear paraffin with regard to explosion, and iso-octane ((Octane Number = 100), highly branched paraffin) highly stable to explosion, as represented in Fig 1a and Fig 1b. A multiple branched paraffin reacts slower than a linear one, due to the higher electronic stability in the ramified skeleton, responsible for a better knock resistance.
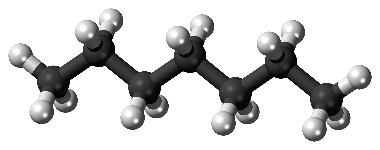
Fig. 1a: n-heptane, RON 0 |
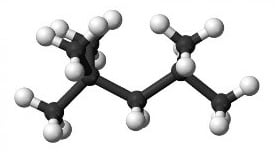
Fig. 1b: iso-Octane, RON 100 |
Nowadays, octane sees an increase in demand. Gasoline RON specification is generally above 90 worldwide. With more stringent regulations implemented worldwide, refiners are looking for solutions with minimum investment to enhance Gasoline Octane, while meeting the targets of low sulphur / olefin / benzene / aromatics contents at the same time.
Most of the refineries are equipped with a Reforming unit, which is dedicated to increase the heavy naphtha octane mainly by converting C7+ compounds into high-RON aromatics. However, aromatics content in gasoline is limited due to their carcinogenic property.
Light Naphtha octane contribution to the pool is usually low (RON 65-75).
However, when looking at C6 compounds for instance, the one with the highest octane number is the 2, 3 DiMethyl Butane (RON 104) vs the straight one , the n-C6 (RON 25), as shown in Fig 2a and Fig 2b. Thus, how to get this RON increase? The response is thanks to a skeleton rearrangement from a linear molecule to a multiple-branched molecule.
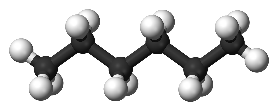
Fig. 2a: n-hexane, RON 25 |
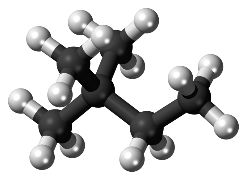
Fig. 2b: 2,3 DMB, RON 104 |
C5/C6 Isomerization is a key unit in a refinery as it boosts the octane value of Light Naphtha, producing a sulfur-free, olefin-free and benzene-free isomerate. The RON can reach up to 92 depending on feed composition and unit scheme. The isomerate is then an ideal blend stream for the gasoline pool formulation.
Several schemes are available and have to be optimized according to the Light naphtha cut composition and the Octane barrel target.
For moderate RON targets, the Once Through scheme enables the reach of 83-84 RON. The scheme with DeIsoHexanizer recycle is a must for higher RON requirement (87-89), especially for a feed with very high content in C6 compounds. Indeed, the low octane C6 are recycled back to the reaction section in order to get several passes isomerization (as shown in the example in Table 1). Further distillation options (DeIsoPentanizer, DePentanizer, etc) for the highest RON targets (92) are selected to maximize C5 cut isomers potential.
| % | Fresh Feed | FF + DIH recycle | RX effluent | Isomerate product from DIH |
|
iC5* / (iC5+nC5) |
40 | 40 | 77 | 77 |
|
iC6** / (iC6+nC6) |
55 | 73 | 89 | 99 |
|
22DMB / (iC6+nC6) |
1 | 3 | 33 | 79 |
|
23 DMB / (iC6+nC6) |
0 | 8 | 10 | 8 |
| DMB / (iC6+nC6) |
1 | 11 | 43 | 87 |
* iC5 = 2,2 dimethylpropane & isopentane
** iC6 = 2,2-DMB ; 2,3-DMB ; 2-MP ; 3-MP
Table 1: Example of DIH recycle benefit in C5/C6 isomerization process (It is worth mentioning that the figures are dependant from DIH operating parameters that are adjusted according to RON target and from unit fresh feed composition).
Paraffins isomerisation reactions are equilibrated reactions. The lower the temperature, the more the thermodynamic equilibrium is pushing towards paraffins isomerisation, and the lower is the cracking.
The activity of Platinum on Chlorinated Alumina catalysts is higher as compared to sulfated zirconia (or metal oxide) catalysts ones. Therefore, efforts were focused to improve this offer even further.
As depicted in Fig. 3, the Isomerization reaction section consists of two reactors in a series, with special valve arrangements allowing each reactor operate in the lead or tail position. Hydrogen utilization is optimized in this once-through scheme, requiring neither a recycle compressor nor a separator drum, which leads to the lowest investment and operating cost at a given octane barrel target.
The chlorinated alumina-type catalyst requires a slight continuous 150-ppm chloriding agent injection in reactor feed to prevent the catalyst from chlorine elution. No corrosion is expected as feed and hydrogen make-up feeding the unit are dried up. Consequently, this chloriding agent is converted into dry HCl in the reaction section, and this slight excess of HCl recovered in the gaseous effluent has to be neutralized with caustic soda. This neutralization represents a small proportion of chemicals consumption as compared to the overall balance in a whole refinery. However, refiners have to comply with more and more environment challenges and are looking for minimizing chemicals while producing the more valuable products possible.
Latest technical innovation in isomerization process has allowed patented improvements such as Axens HCl Recovery Innovation consisting of a better environment-friendly technical solution: it leads to minimize the chloriding agent injection and consequently the caustic soda requirement.
This novelty consists in recovering the major part of Stabilizer off gas rich in HCl (usually sent directly to a Caustic Scrubber), and recycling it to the reaction section.
Fig. 3: Scheme with DIH recycle and HCl recovery section
A simple absorption section is added to the conventional scheme. The stabilizer off gas, rich in HCl, is recontacted with a fraction of the reaction feedstock. The resulting absorber off-gas presents a lower HCl content as compared to the net Stabilizer off gas and at the same time, the reaction section feedstock is enriched in HCl, thus drastically decreasing the chloriding agent injection.
To this day, 16 units have been designed with the HCl recovery section.
Among those references, Dongying Qirun Petrochemical Co. Ltd. (Dongying, Shandong, China) and Jiangsu Xinhai Petrochemicals Co. Ltd. (Lianyungang, Jiangsu, China) are the first two licensees to operate this patented Axens innovation.

Qirun and Jiangsu Xinhai units started up in 2018-2019. Their 2 to 3 years of operation provides enough hindsight for them to assess the positive results of this section addition.
The table below (Table 2) provides a comparison of Qirun and Jiangsu Xinhai ISOM units, when operating with and without the HCl recovery section.
|
Base case without HCl recovery |
With HCl recovery section | |||
|
|
Qirun | Jiangsu Xinhai | ||
| Scheme | Once through | DIH recycle | ||
| Chloriding agent | kg/day | Base | Base - 70% | Base - 75% |
| Caustic soda (pure) | kg/day | Base | Base - 70% | Base - 75% |
| Total H2 consumption | kg/h | Base | Base - 2.1% | Base - 1.9% |
| Isomerisation section Yield | %vol | Base | Base + 0.9% | Base + 1.0% |
| RON | Base | Base + 0.2 | Base + 0.2 | |
| RVP | psi abs | Base | Base + 1% | Base + 1% |
| RON barrel | Base | Base + 1.1% | Base + 1.2% | |
Table 2: Qirun and Jiangsu Xinhai ISOM units operating figures with and without HCl recovery

ISOM unit director
Jiangsu Xinhai Petrochemicals Co. Ltd.
« The start-up went very smoothly in 2019, even when the feed flowrate fluctuated between 65% to 105% of the design flowrate.
Axens process design is quite robust to face possible start-up and operating troubles. After a week of operation, the temperatures were adjusted as per design to 113°C and 120°C for First and Second Reactors respectively. The isomerate RON was above 88, up to 89.2 sometimes, according to our laboratory.
HCl Absorber efficiency was high. C2Cl4 injection was gradually decreased to minimum recommended ratio of Cl/feed but HCl concentration detected in Stabilizer off-gas was still above 5000 ppm.
Currently we are still very satisfied with Axens ISOM technology and ATIS-2L catalyst. Thanks to the HCl recovery innovation we save 75% of chemicals, and earn 1% isomerate yield more. »

ISOM unit director
Dongying Qirun Petrochemical Co. Ltd.
« The unit has been started-up within 6 weeks. Reactors were oiled-in one by one (R-202 on January 2nd and R-203 on January 4th 2019). Then HCl absorber was put in service on January 7th.
With HCl absorber, C2Cl4 flow rate was decreased, while HCl content in Stabilizer off-gas remains higher than 3000 ppm.
We believe Chemicals consumption could be saved by more than 75%, but we are currently limited by the minimum turndown flowrate of the selected chloriding agent pump.
Axens and Qirun staff worked jointly very satisfactorily to start-up, operate and follow successfully the unit. »
The industrial operation confirms the predictions regarding the benefits of such section.
While Qirun and Jiangsu Xinhai sites have benefited from this innovation since the design stage, this solution can also be provided as a plug & play system, that allows to drastically reduce chemicals consumption combined with octane-barrel improvement.
It is now possible, for a mature process, to minimize chemicals consumption and side effluents, while producing a high-quality base to blend in the gasoline pool, which has then a green color when speaking about products valorization.
Did you know? Axens has:
Lead Gasoline Technologist
Axens
Elodie Letourmy is a Lead Gasoline Technologist in Axens Technology and Technical Support Business Division. She joined Axens in 2003 as a process design engineer, and became a project manager for Isomerization and naphtha hydrotreatment projects. During five years she has trained Licensees to understand their new unit, to start it up, operate it (on site, on simulator) and spent almost one year as start-up advisor in India for NHT and Isomerization units. She joined the technology group in 2014 where she achieved Technical proposals related to Gasoline pool technologies. Mrs. Letourmy earned an engineering degree from the Institut National des Sciences Appliquées, along with a post-graduate engineering degree from the IFP School, France.
Tech. service engineer
Axens, Beijing, China
Binglin Liu joined Axens Beijing office in 2015 as a Tech. service engineer, mainly in charge of units start up and follow up for reforming and ISOM. Mr. LIU earned an engineering degree from China University of petroleum and has experience in Sinopec and Honeywell for different roles before Axens.
ISOM unit director
Jiangsu Xinhai Petrochemicals co. Ltd., China
Yongwang SHANG graduated from Weifang Vocational College, he has been working for 15 years in Xinhai petrochemical so far, in charge of the operation and management for reforming and ISOM unit.
ISOM unit director
Dongying Qirun Petrochemical Co. Ltd., China
Rongguang SONG has 21-year experience with Qinrun. He has been working for different units including hydroprocessing, reforming and ISOM.
Product Line Team Manager
Axens, Rueil-Malmaison, France
Laurent Watripont is a Clean Fuels Technologies Director expert and Product Line Team Manager in the Isomerization / Alkylation / Oligomerisation / Alcohol To Jet Product line of Axens Technology and Technical Support Business Division. He joined IFPEN in 1994 where he served successively as a Process Engineer and a Technical Proposal Engineer. Since the creation of Axens in 2001, he belongs to the group in charge of the gasoline pool problematics, is in charge of the expertise for isomerization process, before attaining his current position.
He holds an Engineering Degree from the Ecole Nationale Supérieure de Chimie in Toulouse, and a Master Degree in Refining and Petrochemicals from the IFP School in Rueil-Malmaison, France.